Rare Psychiatry News
Advertisement
Spotlight On
Lennox-Gastaut syndrome
Lennox-Gastaut syndrome (LGS) is a rare but severe form of childhood epilepsy, characterized by a triad of multiple seizure types, characteristic electroencephalogram (EEG) findings, and intellectual impairment
Rare View
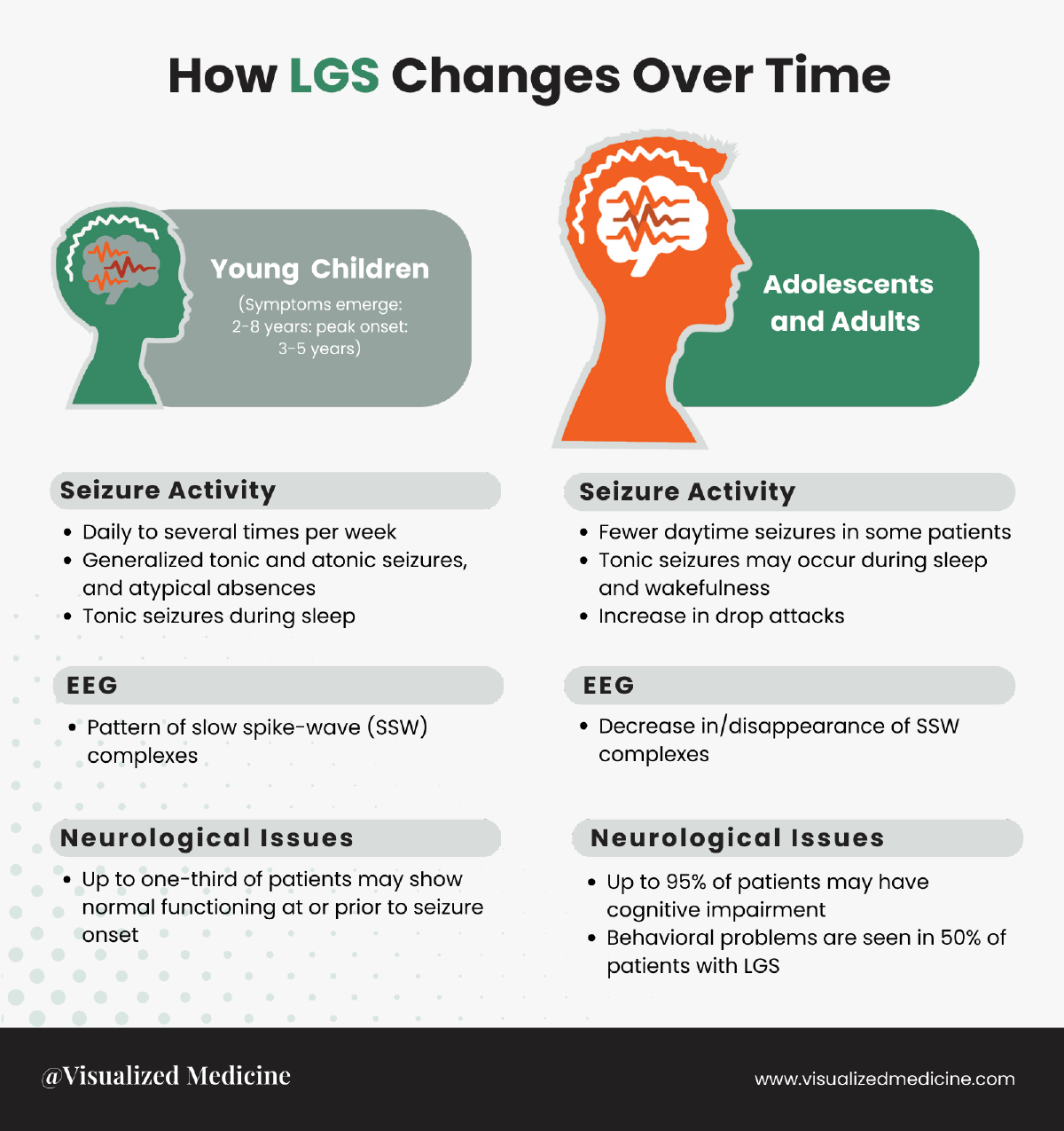
Lennox-Gastaut Syndrome (LGS) is a severe form of childhood epilepsy characterized by multiple seizure types, cognitive impairment, and specific electroencephalogram (EEG) patterns. The impact of LGS on individuals tends to evolve as patients age, leading to changes in the clinical presentation and challenges faced. Understanding the evolving nature of LGS is crucial for accurate diagnosis and tailored therapeutic interventions, optimizing patient care.
5 Facts you should know
FACT
Lennox-Gastaut syndrome (LGS) is a pediatric epilepsy syndrome characterized by multiple seizure types; intellectual impairment or regression.
FACT
A hallmark sign of LGS is “drop seizures”, which may occur several times/day.
FACT
The mean age at epilepsy onset is 26-28 months.
FACT
LGS has characteristic abnormal findings on electroencephalogram (EEG), with paroxysms of fast activity and generalized slow spike-and-wave discharges (1.5-2 Hz).
FACT
A significant correlation exists between age of onset of seizures and mental deterioration.
Advertisement
Interest over time
Google searches
Common signs & symptoms
Multiple Seizure Types
- Tonic seizures, resulting in sudden muscle stiffness and falls
- Atonic seizures (drop attacks) involving abrupt loss of muscle tone and falls
- Absence seizures marked by brief altered consciousness and staring spells
- Myoclonic seizures presenting as brief, shock-like muscle jerks
Cognitive Impairment
- Substantial intellectual disability impacting cognitive abilities
- Learning difficulties and challenges in acquiring and retaining information
- Behavioral issues, including aggression and hyperactivity
Distinctive EEG Patterns
- Slow spike-wave complexes at 2.5 Hz and generalized paroxysmal fast activity (GPFA) are characteristic EEG findings
Early Onset and Childhood Presentation
- Onset typically in early childhood
Drug-Resistant Epilepsy
- Often exhibits poor responsiveness to traditional antiseizure drugs
Psychiatric Comorbidities
- Coexistence of various behavioral and psychiatric disorders, including ADHD, anxiety, and mood disorders
Risk of Physical Injuries
- Frequent falls during seizures elevate the risk of injuries
Regression of Skills
- Loss of developmental milestones, such as language and motor skills
Sleep Disturbances
- Seizures, particularly tonic and atonic types, may predominantly occur during sleep
References:
Arzimanoglou, A., French, J., Blume, W., Cross, J. H., Ernst, J. P., Feucht, M., ... & Vining, E. P. (2009). Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. The Lancet Neurology, 8(1), 82-93. Wheless, J. W., & Kim, H. L. (2014). Lennox-Gastaut syndrome: a state of the art review. Epilepsia, 55(S4), 3-9. International League Against Epilepsy (ILAE) Guidelines and Recommendations: https://www.ilae.org/guidelines. Epilepsia - The official journal of the ILAE: https://onlinelibrary.wiley.com/journal/15281167.
Current treatments
Cannabidiol (Brand name: EPIDIOLEX®)
Manufactured by Jazz Pharmaceuticals, Inc.
FDA-approved in 2018, EPIDIOLEX is a plant-derived cannabis-based medicine administered as an oral solution that contains highly purified cannabidiol (CBD). Epidiolex is structurally distinct from other AEDs with efficacy in multiple seizure types across TSC, LGS, and Dravet syndrome. Epidiolex is the only FDA-approved treatment indicated for seizures associated with TSC, LGS, and Dravet syndrome in patients 1 year and older.
CBD is a chemical component of the Cannabis sativa plant. However, CBD does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC). It is THC (and not CBD) that is the primary psychoactive component of cannabis.
Rufinamide(Brand name: Banzel)
Manufactured by Eisai Medical Research, Inc.
FDA-approved indication: Adjunctive therapy of seizures associated with Lennox-Gastaut syndrome.
Felbamate(Brand name: Felbatol®)
Manufactured by Meda Pharmaceuticals Inc.
FDA-approved indication: As adjunctive therapy in the treatment of partial and generalized seizures associated with the Lennox-Gastaut syndrome in children.
Perampanel(Brand name: Fycompa)
Manufactured by Eisai, Inc.
FDA-approved indication: Treatment of Lennox-Gastaut Syndrome. Treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy 12 years of age and older and as adjunctive therapy for the treatment of primary generalized tonic-clonic seizures in patients with epilepsy 12 years of age and older.
Lamotrigine(Brand name: Lamictal®)
Manufactured by Glaxo Wellcome Research and Development. FDA-approved indication: Adjunctive treatment of Lennox-Gastaut syndrome in pediatric and adult patients.
Topiramate(Brand name: Topamax®)
Manufactured by Ortho-McNeil Neurologics
FDA-approved indication: As adjunctive therapy in patients two years and older with seizures associated with Lennox-Gastaut syndrome.
Fenfluramine (Brand name: Fintepla®)
Manufactured by Zogenix International Limited.
The mechanisms by which fenfluramine exerts its therapeutic effects in the treatment of seizures associated with LGS are unknown. Fenfluramine and the metabolite, norfenfluramine, increase extracellular levels of serotonin through interaction with serotonin transporter proteins, and exhibit agonist activity at serotonin 5HT-2 receptors.
Top Clinical Trials
| Title | Description | Phases | Status | Interventions | More Information |
|---|---|---|---|---|---|
| Assessment of Adjunctive Cannabidiol Oral Solution (GWP42003-P) in Children With Tuberous Sclerosis Complex (TSC), Dravet Syndrome (DS), or Lennox-Gastaut Syndrome (LGS) Who Experience Inadequately-controlled Seizures | This study will be conducted to evaluate the safety, pharmacokinetics (PK), and efficacy of adjunctive GWP42003-P in participants \< 2 years of age with tuberous sclerosis complex (TSC), Lennox-Gastaut syndrome (LGS), or Dravet syndrome (DS). | PHASE 3 | RECRUITING | DRUG: GWP42003-P | More Info |
| Investigate Efficacy and Safety of Carisbamate as Adjunctive Treatment for Seizures Associated With LGS in Children and Adults | The primary objective is to evaluate the efficacy of carisbamate (YKP509) as adjunctive treatment in reducing the number of drop seizures (tonic, atonic, and tonic-clonic) compared with placebo in pediatric and adult subjects (age 4-55 years) diagnosed with Lennox Gastaut Syndrome (LGS). | PHASE 3 | RECRUITING | DRUG: Carisbamate | More Info |
| RNS System LGS Feasibility Study | To generate preliminary safety and effectiveness data for brain-responsive neurostimulation of thalamocortical networks as an adjunctive therapy in reducing the frequency of generalized seizures in individuals 12 years of age or older with Lennox Gastaut Syndrome (LGS) who are refractory to antiseizure medications. The intent is to determine the feasibility and the optimal design of a subsequent pivotal study in order to expand the indication for use for the RNS System as a treatment for patients with medically intractable LGS. | PHASE 2 | RECRUITING | DEVICE: RNS System | More Info |
| A Study of Soticlestat as an Add-on Therapy in Children and Adults With Dravet Syndrome or Lennox-Gastaut Syndrome | The main aim of the study is to learn if soticlestat, when given as an add-on therapy, reduces the number of seizures in children and adults with Dravet Syndrome (DS) or Lennox-Gastaut Syndrome (LGS). Participants will receive their standard anti-seizure therapy, plus tablets of soticlestat. There will be scheduled visits and follow-up phone calls throughout the study. | PHASE 3 | RECRUITING | DRUG: Soticlestat | More Info |
| Open-label, Long-term Safety Study of LP352 in Subjects With Developmental and Epileptic Encephalopathy | The objective of this study is to assess the long-term safety, tolerability, and efficacy of adjunctive therapy of LP352 in subjects with developmental and epileptic encephalopathies who completed participation in Study LP352-201. | PHASE 2 | RECRUITING | DRUG: LP352 | More Info |
Top Treatments in Research
| Agent | Class/Mechanism of Action | Development Status | Company | Clinical Studies | More Information |
|---|---|---|---|---|---|
| DRUG: GWP42003-P | As is the case for many other AEDs, the exact MOA by which CBD produces its anticonvulsant effects is unknown. Cannabidiol is a structurally novel anti-convulsant. Cannabidiol does not exert its anti-convulsant effects through CB1 receptors, nor through voltage-gated sodium channels. CBD may exert a cumulative anti-convulsant effect, modulating a number of endogenous systems including, but not limited to neuronal inhibition (synaptic and extrasynaptic GABA channels), modulation of intracellular calcium (TRPV, VDAC, GPR55), and possible anti-inflammatory effects (adenosine). CBD does not directly bind to, nor activate, CB1 and CB2 receptors at concentrations pharmacologically relevant to its anticonvulsant effect. Among the likely mechanisms of action, modulation of intra-cellular calcium via GPR-55, TRPV, and VDAC is under active investigation in our research laboratories. Additional mechanisms under exploration by our researchers include adenosine modulation, glycine and GABAergic modulation, and serotonin agonism. | PHASE 3 | Jazz Pharmaceuticals | More Info | More Info |
| DRUG: Carisbamate | Carisbamate is a novel neuromodulator whose primary mechanism of action remains poorly understood. It exhibits a broad spectrum of efficacy in animals and may also possess disease-modifying effects by reducing neuronal loss. | PHASE 3 | SK Life Science, Inc. | More Info | More Info |
| DRUG: Soticlestat | Soticlestat is a potent, highly selective, first-in-class inhibitor of the enzyme cholesterol 24-hydroxylase (CH24H), with the potential to reduce seizure susceptibility and improve seizure control. CH24H is predominantly expressed in the brain, where it converts cholesterol into 24S-hydroxycholesterol (24HC) to adjust the homeostatic balance of brain cholesterol. 24HC is a positive allosteric modulator of the NMDA receptor and modulates glutamatergic signaling associated with epilepsy. | PHASE 3 | Takeda | More Info | More Info |
| DRUG: Bexicaserin | Bexicaserin (LP352) is an oral, centrally acting, 5-HT2C superagonist in development for the potential treatment of seizures associated with DEEs such as Dravet syndrome, Lennox-Gastaut syndrome (LGS), tuberous sclerosis complex (TSC), CDKL5 deficiency disorder (CDD), and other epileptic disorders. Bexicaserin is designed to modulate GABA and, as a result, suppress the central hyperexcitability that is characteristic of seizures. | PHASE 2 | Longboard Pharmaceuticals | More Info | More Info |
Caregiver Corner
Tools and resources for your patients living with LGS

Seizure disorder treatment centers
Hover over the pins to see each center. Click on the location to navigate to their website.
Please note that this is not an exhaustive list and there may be other treatment centers in the United States that are not included. It is also important to consult with a medical professional to determine the best treatment plan for each individual case.
Tools and resources

Dedicated to improving the lives of individuals impacted by LGS
New Family Welcome Kit - Contains educational material about LGS including programs offered by the LGS Foundation, where to turn for additional help, and information on other useful resources. New Family Welcome Kits are free to LGS families and caregivers.
Navigating the LGS Maze Newsletter - Keeps you up to date with the latest information on current research, our community, our programs, the grants we award, and upcoming fundraisers & special events.
Real Stories From Real Families - Discover the stories of real people who live with Lennox-Gastaut syndrome (LGS), Dravet syndrome, and tuberous sclerosis complex (TSC).
The Care Giver Series - Join actor Greg Grunberg as he travels around the country, sharing the stories of caregivers of families living with Lennox-Gastaut syndrome (LGS), Dravet syndrome, and tuberous sclerosis complex (TSC), and giving them a much-needed day of care.
The LGS Patient Experience:
Learn from more than 1,300 LGS caregivers

Get years of knowledge and experience condensed into a simple report.
TSC Download Report
"*" indicates required fields
Other resources
Click the drop-down to see info on Patient Advocacy Groups and a Glossary of common terms